Answer: 250 mL
Explanation: %(m/v) stands for mass by volume percentage. 2.5%(m/v) means 2.5 grams of a solute present in 100 mL of a solution.
The question asks to calculate the volume of 2.5%(m/v) KOH solution that can be prepared from 125 mL of a 5.0%(m/v) KOH solution.
It's a dilution problem and could easily be solved by using dilution equation:
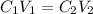
where
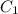 is the concentration before dilution and
is the concentration before dilution and
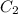 is the concentration after dilution. Similarly,
is the concentration after dilution. Similarly,
 is the volume before dilution and
is the volume before dilution and
 is the volume after dilution.
is the volume after dilution.
Let's plug in the values in the equation:
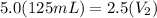
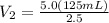
 = 250 mL
= 250 mL
So, 250 mL of 2.5%(m/v) KOH solution can be prepared from 125 mL of 5.0%(m/v) KOH solution.