Answer : The solubility product expression is,
![K_(sp)=[Hg^+]^2[I^-]^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/krwl54znjs9xm6z6mtf0si8p725j9w0ucg.png)
Explanation :
Solubility product constant : It is defined as the product of the concentration of the ions present in a solution raised to the power by its stoichiometric coefficient in a solution of a salt. This takes place at equilibrium only. The solubility product constant is represented as,
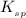 .
.
The dissociation of mercury(i) iodide is written as:

The expression for solubility constant for this reaction will be,
![K_(sp)=[Hg^+]^2[I^-]^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/krwl54znjs9xm6z6mtf0si8p725j9w0ucg.png)
Therefore, the solubility product expression is
![K_(sp)=[Hg^+]^2[I^-]^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/krwl54znjs9xm6z6mtf0si8p725j9w0ucg.png)