Answer: An increase in the amount of radium-226 will increase the reaction rate as radioactive decay follows first order kinetics.
An increase in the amount of
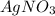 and NaCl will increase the reaction rate as the reaction follows first order kinetics with respect to
and NaCl will increase the reaction rate as the reaction follows first order kinetics with respect to
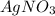 and first order with respect to NaCl.
and first order with respect to NaCl.
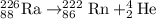
![rate= k[Ra]^1](https://img.qammunity.org/2019/formulas/chemistry/college/aapgllk8gbcxy47o558pewarw48wi4yu07.png)
If the amount of radium is doubled , the rate of the reaction also doubles.
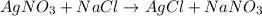
![rate= k[AgNO_3]^1[NaCl]^1](https://img.qammunity.org/2019/formulas/chemistry/college/r33mu4em0n9k5zo6lwpw6r43c5bzqlcazp.png)
If only the amount of
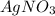 is doubled , the rate of the reaction also doubles. If only the amount of NaCl is doubled , the rate of the reaction doubles. lf the concentration of
is doubled , the rate of the reaction also doubles. If only the amount of NaCl is doubled , the rate of the reaction doubles. lf the concentration of
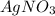 and NaCl both are doubled, the reaction rate becomes 4 times.
and NaCl both are doubled, the reaction rate becomes 4 times.