Step-by-step explanation:
A covalent bond is defined as the bond which is formed by sharing of electrons.
For example, atomic number of carbon is 6 and its electronic distribution is 2, 4. Whereas atomic number of hydrogen is 1.
Hence, in order to complete their octet a carbon atom needs 4 more electrons whereas a hydrogen atom needs one more electron.
Therefore, both carbon and hydrogen atom will combine together and result into the formation of
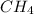 that is, methane.
that is, methane.
Therefore, we can conclude that the chemical compound seen here is called methane. Carbon and hydrogen are chemically combined using covalent chemical bonds in which electrons are shared.