Answer: The molarity of the solution of barium hydroxide is 0.5 mol/L.
Step-by-step explanation:
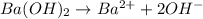
Given , concentration of hydroxide ion =
![[OH^-]=1.0 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/6v58g671t9edve5xsfyy6qav8pfkge37c5.png)
1.0 M of hydroxide ion means that 1 mole of hydroxide ion is present in 1 L solution.
According to reaction 1 mole of barium hydroxide gives 2 moles of hydroxide ions.
Then, 1 mole of hydroxide ion will be given by:
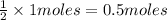 of barium hydroxide
of barium hydroxide
Since, there are 1 mole of hydroxide ions present 1 L of water and 0.5 moles of barium hydroxide in 1 L.
So, the molarity of the solution of barium hydroxide is 0.5 mol/L.