Answer: The correct answer is 148.33 grams.
Step-by-step explanation:
To calculate the molecular weight of a compound, we add on the mass of individual atoms are added multiplies with the number of individual atoms present.
Calculating the mass of
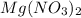 :
:
Mass of magnesium = 24.305 g/mol
Mas of nitrogen = 14.007 g/mol
Mass of oxygen = 15.999 g/mol
Mass of
![Mg(NO_3)_2=[24.305+(2* 14.007)+(6* 15.999)]=148.33g/mol](https://img.qammunity.org/2019/formulas/chemistry/high-school/4uo2rsztr5j0vhytfq3exzrjlhmzvk0ajc.png)
1 mole of magnesium nitrate has a mass of 148.33 grams.
Hence, the correct answer is 148.33 grams.