1) A light wave consists of many photons, each photon carrying an energy equal to
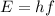
where h is the Planck constant and f is the light frequency, so the energy of the wave is given by the frequency of the light.
The intensity of a light wave is instead the power P developed by the wave per unit of surface A:
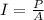
where the power is actually the ratio between the total energy of the wave and the time:
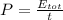
and so, the intensity is
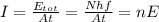
where N is the total number of photons emitted (and so, the total energy is just the number of photons N times the energy of a single photon E), and
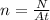
is the number of photons emitted per unit of time and unit of area.
2)
How would you change the energy?
As we saw in point 1), the energy of the light is given by
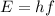
So, we can see that we can change the energy of the light wave by changing its frequency, f.
3) How would you change the intensity?
The formula for the intensity was:
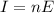
and so, we can see that we can change the light intensity either by changing the number of photons emitted per seconds per squared meters (n), or by increasing the energy of the photons E (so, by changing the frequency of the light)
4) Are these changes independent of one another? Why or why not?
They are not completely independent, because we see from the previous formula that the intensity also depends on the energy E. However, it is possible to change energy without changing the intensity, and viceversa, looking again at the two formulas:
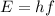
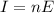
- we can change the energy by changing the frequency of the light, and we can change the number of photons emitted n such that the product nE remains constant, so the intensity I remains constant.
- we can change the number of photons emitted n, so the intensity I changes, but we can keep the frequency of the light constant, so that the energy does not change.