Answer:5.86 kg sodium aluminate is required to produce 5.01 kg of
![Na_3[AlF_6]](https://img.qammunity.org/2019/formulas/chemistry/college/s18jadhcnn29skjc7yqhsovdgiejt3z77i.png) .
.
Step-by-step explanation:
![3NaAlO_2+6HF\rightarrow Na_3[AlF_6]+Al_2O_3+3H_2O](https://img.qammunity.org/2019/formulas/chemistry/college/ff7pbbwdisuyip6gtadk59hooidnml5efe.png)
Mass of
![Na_3[AlF_6]=5.01kg=5010g](https://img.qammunity.org/2019/formulas/chemistry/college/ysorl8nd52r84rmtg06gpw0gc0200k7uc7.png) (1kg=1000g)
(1kg=1000g)
![\text{Moles of }Na_3[AlF_6]=\frac{\text{Given mass of}Na_3[AlF_6]}{\text{molecular mass of}Na_3[AlF_6]}=(5010 g)/( 209.94g/mol)=23.86 moles](https://img.qammunity.org/2019/formulas/chemistry/college/8efw6uwrw24hzdeyskwgfxi8r1jsrgu8i7.png)
According to reaction, 1 mol of
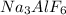 is obtained from 3 moles of
is obtained from 3 moles of
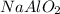 , then 23.86 moles of
, then 23.86 moles of
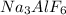 will be obtained from:
will be obtained from:
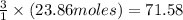 moles of
moles of
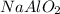
Mass of sodium aluminate is required to produce 5.01 kg of
![Na_3[AlF_6]](https://img.qammunity.org/2019/formulas/chemistry/college/s18jadhcnn29skjc7yqhsovdgiejt3z77i.png) .
.
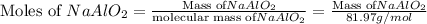
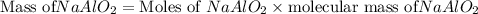
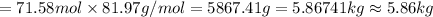
5.86 kg sodium aluminate is required to produce 5.01 kg of
![Na_3[AlF_6]](https://img.qammunity.org/2019/formulas/chemistry/college/s18jadhcnn29skjc7yqhsovdgiejt3z77i.png) .
.