By using heat of fusion (6.02 kJ / mol) of water as a conversion factor to determine the moles of water then use molar mass (18.02 g/mol) of water as a conversion factor to determine the mass of water and finally use the mass of one ice cube as a conversion factor to determine the number of ice cubes that absorbs 67.1 kJ energy:
67.1 kJ *
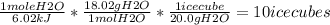
So the correct answer is 10 ice cubes