Answer:
73.10% the percent purity of aspirin.
Step-by-step explanation:
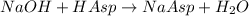
Moles of NaOH:

Moles of NaOH = 0.001002 moles
According to reaction. 1 NaOH reacts with 1 mole of aspirin .
Then 0.001002 moles NaOH will reacts with :
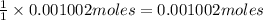
Mass of 0.001002 moles of aspirin:
= 0.001002 moles × 180.2 g/mol = 0.18056 g
Percentage of purity of aspirin:
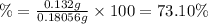
73.10% the percent purity of aspirin.