The Balanced chemical equation of reaction of Borane with oxygen is as follow,
B₂H₆ + 3O₂ -----> 2HBO₂ + 2H₂O
According to this equation 27.66 g (1 mole) of B₂H₆ reacts with oxygen to produce 36 g (2 moles) of water.
The amount of water produced when 19.2 g of B₂H₆ reacted is calculated as follow,
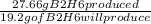
=
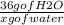
Solving for x,
x = (36 g of H₂O ₓ 19.2 g of H₂B₆) / 27.66 g of B₂H₆
x =
24.98 g of H₂O
Result:
24.98 g of water is produced when 19.2 g of B₂H₆ is reacted with excess of oxygen.