Radioactive decay is expressed by the following formula:
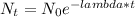
N₀ is the initial number of undecayed atoms.
Nt is the number of undecayed atoms remaining after time t
λ is the decay constant.
The relationship between λ and the half life time t1/2 is:
λ =
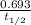
= 0.693 / 5.27 = 0.1315
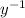
Taking natural logs for both sides of the decay expression:
ln Nt = ln N₀ - λ t
ln Nt = ln 199 - (0.1315 x 20)
= 5.293 - 2.63 = 2.66
From which:
Nt = 14.29 g