We have:
V = 55.0 gallons x 3.78541 = 208 L
P = 16500 kPa
T = 23 + 273.15 = 296.15 K
Part A) From the equation PV =
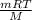
we get:
mass of O₂ = (16500 kPa x 208 L x 32 g/mol) / (8.314 x 296.15 K)
= 44.6 Kg
Part B) at STP we have:
T = 273.15 K and P = 101.3 kPa
so from PV = mRT / M
V = (44600 x 8.314 x 273.15) / (32 x 101.3)
= 31248 L
Part C) From the equation PV = mRT / M
we get
T = (150 atm x 101.3 kPa / atm x 208L x 32g/mol) / (8.314 x 44600)
= 272.8 K
Part D) we have:
T = 24 + 273.15 K = 297.15 K
so from PV = mRT / M, we get
P = (44600 x 8.314 x 297.15) / (32 x 55)
= 62464 kPa