Answer:
1.0 moles of
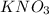 are required to make 0.50 liters of a 2.0 m solution of
are required to make 0.50 liters of a 2.0 m solution of
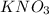 .
.
Step-by-step explanation:
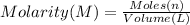
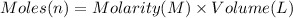
Moles of potassium nitrate = n
Volume of potassium nitrate solution = 0.50 L
Molarity of the potassium nitrate = 2.0 M

1.0 moles of
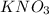 are required to make 0.50 liters of a 2.0 m solution of
are required to make 0.50 liters of a 2.0 m solution of
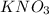 .
.