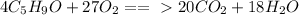When you balance a reaction, you want the same number of atoms of each element on both sides of the arrow/in the reactants (left side) and products (right side). For example, if there are four oxygen on the left, you want four oxygen on the right and if there are three hydrogen on the left, you want three hydrogen on the right, etc.
So your equation is:
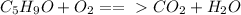
On the left, you have 5 carbon, 9 hydrogen, and 3 oxygen.
On the right, you have 1 carbon, 2 hydrogen, and 3 oxygen.
You want the same numbers of each on both sides. To do this you have to multiply the compounds by adding numbers to the front of them. You can never add subscripts. Most of the time, you are reasonably guessing and checking to see which numbers work, concentrating on one element at a time.
I balanced the H's first, since there are 9 on the left and 2 on the right. You want a number that is a multiple of both. I tried 18 at first, meaning
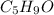
had to be multiplied by 2 and

by 9. However, I ran into trouble with the O's because that meant I would end up with an even number of O's on the left, no matter what I multiplied

by, and an odd number of O's on the right.
That meant I would have to use 36 as my next least common multiple of 9 and 2, giving
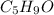
a coefficient of 4 and

a coefficient of 18. Everything else balanced out from there. There were 20 C's on the right because of
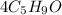
(from the left). And

had a coefficent of 27 because there were already 4 on the left, and 58 on the right, so 58-4 = 57, divided by 2 (2 oxygen atoms in

) = 27.
The final equation was: