Answer:
2.78 mL of solution will be needed to make 4.5 M solution with 0.50 grams of NaOH.
Step-by-step explanation:
Moles of sodium hydroxide = n
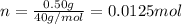
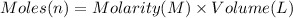
Volume of sodium hydroxide solution =V
Molarity of the sodium nitrate solution = 4.5 M
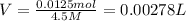
V = 0.00278 L = 2.78 mL
2.78 mL of solution will be needed to make 4.5 M solution with 0.50 grams of NaOH.