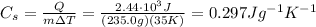When an object absorbs an amount of energy equal to Q, its temperature raises by

following the formula
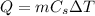
where m is the mass of the object and
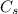
is the specific heat capacity of the material.
In our problem, we have
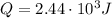
,
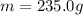
and
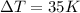
, so we can re-arrange the formula and substitute the numbers to find the specific heat capacity of the metal: