The elements found in the Group 1, or the Alkali Metal Group, have electronic configurations that end in
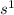
.This means that they have 1 electron readily available to release in order to achieve a stable state.
When these atoms release the valence electron, they will achieve a stable state. For example, Lithium's stable state will be
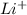
and Sodium will be
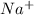
.
The oxidation state will then be +1.
The answer is C.