For this question, since the parameters volume, pressure and temperature changes, we can use the combined gas law equation.
It states that PV/T = constant
Therefore,
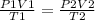
Where parameters for the first instance are given on the left side and parameters for the second instance are given on the right side of the equation
Temperature in K - T1 = 6 °C + 273 = 279 K
T2 = 34 °C + 273 = 307 K
substituting the values in the equation
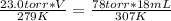
V = 55.5 mL