Heat absorbed (q) = 0.185 kJ
Molar heat of vaporization (ΔH) = 24.7 kJ/ mole
molar mass of chloroethane = 64.51 g/mole
q = (ΔH) (n) where n is number of moles
n =
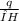
= [tex] \frac{0.185}{24.7} = 7.5 x 10⁻³ mole
number of moles = mass (g) / molar mass
mass (g) = number of moles x molar mass
= (7.5 x 10⁻³) x (64.51) = 0.48 g