Answer:0.0082 moles of
 will be needed to neutralize the 0.0164 KOH.
will be needed to neutralize the 0.0164 KOH.
Step-by-step explanation:
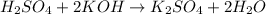
2 moles of Potassium hydroxide neutralizes 1 mole of sulfuric acid.
Then 0.0164 moles of potassium hydroxide will neutralize
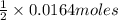 of sulfuric acid that is 0.0082 moles.
of sulfuric acid that is 0.0082 moles.
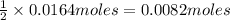
0.0082 moles of
 will be needed to neutralize the 0.0164 KOH.
will be needed to neutralize the 0.0164 KOH.