Answer:
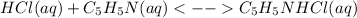
Step-by-step explanation:
Hello,
In this case, as the pH must remain less than 7 we must look for a strong acid and a weak base with a 1 to 1 mole relationship in the chemical reaction since equal molar amounts are to be mixed. This is due to the fact the the whole acid will dissociate and as the weak base does not completely dissociate, there will be remaining hydride ions causing the required pH. In such a way, the best combination is hydrochloric acid (strong acid) with pyridine (weak base) because they have the aforesaid mole relationship and there will be unreacted hydride ions causing a less than 7 pH considering the reaction:
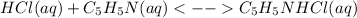
Best regards.