Answer: The mass of iron present is 55.40 g
Step-by-step explanation:
We know that:
Molar mass of iron (III) oxide = 159.7 g/mol
Molar mass of iron = 55.85 g/mol
We are given:
Mass of iron (III) oxide = 79.2 g
To calculate the mass of iron in given amount of iron (III) oxide, we use unitary method:
In 159.7 grams of iron (III) oxide, the mass of iron is (2 × 55.85) = 111.7 g
So, in 79.2 grams of iron (III) oxide, the mass of iron will be =
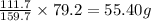
Hence, the mass of iron present is 55.40 g