Answer:
Step-by-step explanation:
Hello,
In this case, based on the given description, the corresponding chemical reaction turns out into:
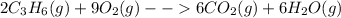
Wherein the numbers preceding the compound formula are referred to the stoichiometric coefficients and the
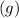 to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.
to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.
Best regards.