The percentage of Chromium in Chromium Oxide is calculated as follow,
Step 1: Calculate Molar mass of Cr₂O₃,
Cr = 51.99 u
O = 16 u
So,
2(51.99) + 3(16) = 103.98 + 48 = 151.98 u
Step 2: Secondly divide molar mass of only chromium with total mass of Cr₂O₃ and multiply with 100.
i.e.
=
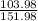
× 100
=
68.41 %
So, the %age composition of chromium in chromium oxide is
68.41 %.