Answer: The percent by mass of water in the hydrate is 45.4 %
Step-by-step explanation:
The chemical equation for the heating of hydrated cobalt (II) chloride follows:
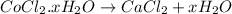
We are given:
Mass of hydrated cobalt (II) chloride = 5.22 g
Mass of anhydrous cobalt (II) chloride = 2.85 g
Mass of water released = (5.22 - 2.85)g = 2.37 g
To calculate the mass percentage of water in the hydrate, we use the equation:
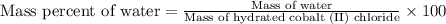
Mass of hydrated cobalt (II) chloride = 5.22 g
Mass of water = 2.37 g
Putting values in above equation, we get:
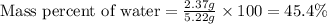
Hence, the percent by mass of water in the hydrate is 45.4 %