Answer: Option (a) is the correct answer.
Step-by-step explanation:
It is known that rule for writing a chemical formula is by cross multiplying the oxidation states of the atoms forming a bond.
So, oxidation state of mercury in
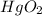 is +4 and oxidation state of oxygen is -2.
is +4 and oxidation state of oxygen is -2.
Therefore, when we cross multiply oxidation states of cation and anion and then we represent in simplest ratio.
Thus, simplest ration in
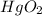 is 1:2.
is 1:2.
Hence, we can conclude that mercury (IV) oxide has molecular formula
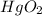 .
.