Answer: The correct answer is
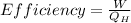 .
.
Step-by-step explanation:
Heat engine: It is a device which converts the heat energy in to the mechanical work. In heat engine, the heat is transferred from high temperature reservoir to the low temperature reservoir.
It is just opposite of the refrigeration.
The expression of the work done is as follows;
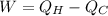
Here,
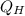 is the heat added to the system and
is the heat added to the system and
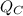 is the heat rejected to the cold reservoir.
is the heat rejected to the cold reservoir.
The formula for calculating the efficiency of a heat engine is as follows;
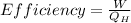
Here, W is the work done and
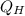 is the heat added from the reservoir which is at high temperature and
is the heat added from the reservoir which is at high temperature and
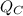 is the heat rejected to the reservoir which is at low temperature.
is the heat rejected to the reservoir which is at low temperature.