The only balanced equation is B. If you look at the equation and break it down you can see that in:
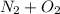
→
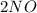
Starting from the left side of the equation there are 2 Nitrogen atoms, and 2 oxygen atoms as indicated by the subscript.
To balance the equation, the number of atoms of each element in the right side equation should be equal to left. By putting the numerical coefficient of 2, you will distribute that to each element. So you will end up with 2 nitrogen atoms and 2 oxygen atoms on the left side of the equation. Thus, the equation is balanced.
The answer again, is B.