Answer: The correct answer is Option C.
Step-by-step explanation:
Ionic bond is formed when complete transfer of electrons takes place from one atom to another atom. The atom which donates electrons is known as electropositive atom and the atom which accepts electron is known as electronegative atom.
This bond is usually formed between a metal and a non-metal.
Sodium is the 11th element of the periodic table with electronic configuration
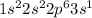
This element will loose 1 electron easily to form stable electronic configuration.
Fluorine is the 9th element of the periodic table with electronic configuration
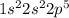
This element will gain 1 electron easily to form stable electronic configuration.
So, sodium will transfer 1 electron to fluorine. Hence, the correct answer is Option C.