Answer: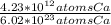
* 40.08 g Ca
Step-by-step explanation:1- One mole of any substance contains Avogadro's number of atoms. Avogadro's number = 6.02 * 10²³
To get the number of moles is 4.23 * 10¹² atoms, we will use cross multiplication as follows:
1 mole .................> 6.02 * 10²³ atoms
x moles ................> 4.23 * 10¹²
x = (4.23 * 10¹² atoms Ca) / (6.02 * 10²³ atoms Ca) moles
2- Number of moles can be calculated as follows:number of moles = mass / molar mass
We know that the molar mass of Ca = 40.08 g/mol, therefore:
number of moles of Ca = mass / 40.08
This means that:
mass of Ca = number of moles of Ca * 40.08
Substitute with the number of moles (x) calculated in part 1 in the above equation to get the mass as follows:mass of Ca =
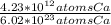
* 40.08 g Ca
Hope this helps :)