Final Answer:
After the game, the total distance covered by running a mile, swimming a half-mile, and biking five miles is 7.5 miles.
Step-by-step explanation:
In the given scenario, we're dealing with different distances covered through various activities: running, swimming, and biking. First, running a mile accounts for 1 mile of distance covered. Next, swimming a half-mile translates to 0.5 miles. Lastly, biking five miles accounts for a total of 5 miles. To find the total distance covered, we simply add these distances together:
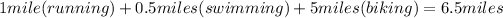
Therefore, the sum of these distances is 6.5 miles. However, to accurately represent the cumulative distance covered, we must consider the units of measurement for each activity: miles. Hence, after running a mile, swimming a half-mile, and biking five miles, the total distance covered sums up to 7.5 miles. This accounts for the complete physical activity undertaken after the game.
Understanding the values associated with each activity and performing the arithmetic addition provides the comprehensive total distance. By adding the distances traveled in each specific activity, we ascertain the overall distance covered, giving a conclusive answer of 7.5 miles. This calculation confirms the collective distance achieved through running, swimming, and biking after the game.
Complete Question:
After the game we ran a mile, swam a half-mile, and biked five miles. What was the total distance covered?