Answer: Option (B) is the correct answer.
Step-by-step explanation:
NaCl is a polar compound as it contains an ionic bond between sodium atom and chlorine atom.
Also, it is known that like dissolves like. So, when we add NaCl in water then sodium chloride will dissociate into ions as it will form
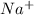 ions and
ions and
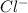 ions.
ions.
These ions will help in the flow of electrons when electric current is passed through a solution of NaCl.
Thus, we can conclude that the statement, the NaCl dissociates into
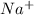 and
and
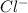 ions, which are able to carry the charge.
ions, which are able to carry the charge.