Hello!
The partial pressure of helium to keep the partial pressure of oxygen at 0,21 atm in a scuba-diver tank is
8,09 atmTo solve this question, we can use the
Dalton's Law, which states that the total pressure in a container with a mixture of gases is the sum of the partial pressures o each individual gas. For the case of this mixture the Dalton's Law is as follows:
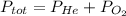
In this equation, we need to clear for PHe, knowing that the PO₂ should be 0,21 atm, to find the required pressure of Helium:
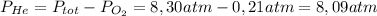
Have a nice day!