Answer:
Image of (R)-2-chloropropanoic acid has been attached below
Step-by-step explanation:
Here longest carbon chain contain 3 carbon atoms (prop means 3 carbon).
Functional group is -COOH (suffix oic acid means presence of -COOH group in longest carbon chain)
Numbering should start by assigning (1) to carbon in -COOH group
One stereocenter present at (2) position.
According to CIP rule of assigning preference to substituents in a stereocenter, Cl(a), -COOH (b), -Me(c) and H(d).
Cl should remain above the plane and H below the plane so that
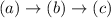 gives clockwise rotation keeping (d) far apart from viewing side.
gives clockwise rotation keeping (d) far apart from viewing side.