Hello!
To prepare 500 mL of 1M Acetic acid from a 17,5M stock solution you'll need to measure
28,57 mL of Acetic Acid to the nearest milliliter and dissolve in a 500-mL flask with water.
A stock commercial solution of acetic acid (
CH₃COOH) has a concentration of 17,5 M. This solution is called
glacial acetic acid. To calculate the volume of Glacial Acetic Acid needed to prepare 500 mL of 1M acetic acid, we'll need to apply the following equation:
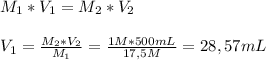
Have a nice day!