Answer: the property of noble gases which is common is that they have a full outer shell of electrons.
Step-by-step explanation:
Noble gases are the gases belonging to Group 18 of the periodic table. The electronic configuration for these gases are:
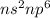
From the electronic configuration, it is visible that they have fully filled outer shell and hence, these gases are least reactive of all the elements in the periodic table.
As, they are not reactive and therefore they do not form any chemical compounds easily.
From the electronic configuration, it is visible that there are no free electrons in these gases, therefore, they are not good conductors of electricity.
Hence, the correct answer is that they all have full outer shell of electrons.