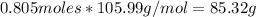Answer:
Mass of Na2CO3 = 85.3 g
Step-by-step explanation:
Volume of solution, V = 750 L
Mass of Ca2+/L = 43 mg
Mass of Ca2+ = 40.08 g
Mass of Na2CO3 = 105.99 g/mol
The net ionic equation between Ca2+ and CO3^2- is:

Based on the stoichiometry:
1 mole of Ca2+ ions combines with 1 mole of CO₃²⁻ ions
Step 1: Calculate moles of Ca2+ in 750 L solution
Mass of Ca2+ in 750 L =
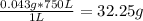
Moles of Ca2+ =
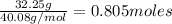
Step 2: Calculate the mass of Na2CO3 required
Moles of Ca2+ = Moles of Co3^2- = 0.805 moles
Mass of Na2CO3 =