Answer:
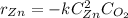
Step-by-step explanation:
Hello,
In this case, the mentioned reaction should be:
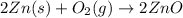
In such a way, considering an irreversible reaction and an elemental reaction, the power of each compound's concentration equals the stoichiometric coefficient in its reaction, thus:
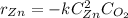
It turns out negative since it is a consumption upon zinc.
Best regards.