Hello!
The right answer is
C) 60 g/LTo know the final concentration of NaI solution, you'll need to calculate the i
nitial amount of NaI in grams in the following way:
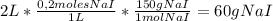
Next, you only need to calculate the concentration on NaI in the new 1 L solution, because only water is evaporating from the initial solution:
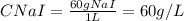
Have a nice day!