Hello!
The volume of the gas when its pressure is increased to 880 mm Hg is
88,64 mLThis question can be easily answered using the
Boyle's Law, which states that as pressure increases, the volume is lower. It can be expressed in a mathematical way as follows:
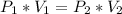
So, from this equation we can clear V₂ to find the volume at 880 mm Hg:
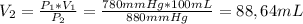
Have a nice day!