Answer:
Heat loss by aluminium will be more as compare to copper.
Step-by-step explanation:
We know that heat lost given as follows
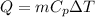
Given that mass of aluminium and copper are same so lets take 1 g.
Lets take the temperature of cold water is 10°C
For aluminium
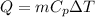
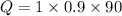
Q= 81 J
For copper
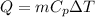
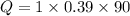
Q= 35.1 J
So from above we can say that heat loss by aluminium will be more as compare to copper.