Answer: 1. a substance with a pH of 3: acid
2. a substance with a pH of 10: base
3. a substance with a pH of 7: neutral
4. an indicator: phenolphthalein
5. a scale that represents the concentration of
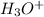 , 0-14: pH scale
, 0-14: pH scale
Explanation:-
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
On the pH scale ranging from 0 to 14 , Acids have pH ranging from 0 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7.
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
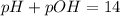
Indicators show a change or appearance in color at particular pH. Phenolphthalein is one such acid base indicator which shows pink color in basic solutions and colorless in acidic solutions.