Answer : Heat release, Q = -5125 J
Explanation :
It is given that,
Mass of liquid copper, m = 25 grams
The heat of fusion of copper, L = 205 J/g
The latent heat of fusion is defined as the amount of heat required to convert solid into liquid phase for one gram of substance.
But in this case, it is required to find the amount of heat released when liquid copper changes to solid.
So, L = - 205 J/g
To find the amount of heat released when 25.0 grams of liquid copper at its freezing point of 1,085°C changes to solid, we can use unitary method.
So,
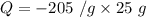
Q = -5125 J
Hence, this is the required solution.