Answer: 24 moles
Explanation:
Combustion is a type of chemical reaction in which hydrocarbon is oxidized to carbon dioxide and water.
The balanced chemical reaction for combustion of butane is :
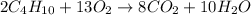
According to stoichiometry,
Given: Oxygen is the excess reagent. Thus butane is the limiting reagent as it limits the formation of product.
2 moles of butane gives 8 moles of carbon dioxide.
Thus 6 moles of butane will give=
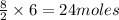 of carbon dioxide.
of carbon dioxide.