for this question we can used the combined gas law equation
for a fixed amount of gas PV/T = constant
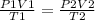
where P - pressure, V-volume and T - temperature
parameters for the first instance are given on the left side and parameters for the second instance are given on the right side of the equation.
Temperature should be in Kelvin
temperature in K = temperature in celcius + 273
T1 = 25.9 °C + 273 = 298.9 K
T2 = -13.6 °C + 273 =259.4 K
substituting the values into the equation , V - volume at the altitude can be found out
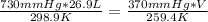
V = 46.1 L