Hello!
The pH of a buffer solution of an acid and its conjugate base is easily calculated using the
Henderson-Hasselbach's equation, with the value for the pKa for the benzoic acid:
(
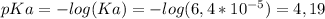
)
Henderson-Hasselbach:
![pH=pKa + log ( ([A^(-)] )/([HA]) ) \\ \\ pH=4,19 + log ( (0,05M)/(0,05M)) \\ \\ pH=4,19](https://img.qammunity.org/2019/formulas/chemistry/high-school/8h3cpm2pcxd0ojbvr627l7j72nhce1eeqm.png)
So, the pH for a 100-mL solution of 0,05 M Benzoic Acid and 0,05 M Sodium Benzoate is
4,19Have a nice day!