Answer : The coefficient of
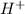 is, 4
is, 4
Explanation :
Balanced chemical reaction : It is defined as the chemical reaction in which the number of individual atoms of an element in reactant side always be equal to the number of individual atoms of an element in product side.
The given balanced half-reaction will be,
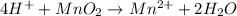
From the balanced half-reaction we conclude that the coefficient of
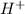 ,
,
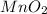 ,
,
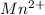 and
and
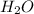 are, 4, 1, 1, 2 respectively.
are, 4, 1, 1, 2 respectively.
Therefore, the coefficient of
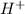 is, 4
is, 4