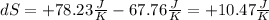From Clausius theorem we get the formula for the change of entropy:
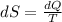
dS is the change of entropy, dQ is the change of energy, and T is the temperature in Kelvin.
We need to convert those temperatures:
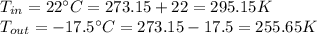
Entropy changes inside and outside:
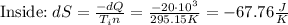
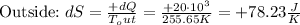
The net entropy increase is: