Answer: The correct answer is Potassium (K).
Step-by-step explanation:
Alkali (Group 1) and Alkaline earth (Group 2) elements react with water to produce metal hydroxide and also releases hydrogen gas.
Reaction of group 1 elements with water:
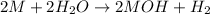
Reaction of group 2 elements with water:
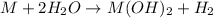
From the given options:
Potassium (K): This metal belongs to Group 1 and readily reacts with water to form potassium hydroxide and hydrogen gas.
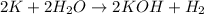
Tin (Sn): This element belongs to Group 14 and does not readily reacts with water.
Nickel (Ni): This element belongs to Group 10 and does not readily reacts with water.
Manganese (Mn): This element belongs to Group 7 and does not readily reacts with water.
Hence, the correct answer is potassium (K).